VIP Newsletter: Spring 2010 Edition
VIP’s mission is to foster communication among teachers of physics and physical science as well as to provide unique learning experiences for teachers and their students.
|
|
| |
Theme: Diffraction
Who: Physical Science teachers and Physics teachers and professors
When: April 24th (time agenda below) 8:30 - 4:00
Where: The physics building, Jesse BeamsLaboratory. There is a good web map at http://www. virginia.edu/webmap/GMcCormickRoadArea.html The physics building is #41. You may want to park behind #38 off of stadium road. Do not park at the physics building. This is 24/7 permit parking. You may be towed is you park next to the physics building.
Why: 'Cause it's a fun way to become better at what you do!
Cost: Free!!!
Sponsor: This meeting is hosted by the Physics Department of the University of Virginia and supported by funds in association with the Virginia Association of Science Teachers and Jefferson Lab.
Agenda
8:30 – 9:00 ...... Hellos and Juice, Coffee, and Danish
9:00 -10:00 ...... Share session: Bring something to share. A demo, 30 copies of a worksheet or lab.
10:00 – 11:00.... Dr. Lindgren (from the University of Virginia) will speak about diffraction labs.
11:00 – 11:30 ... VIP business (Officer elections and VAST participation)
12:45 – 3:00...... Make and Take wave tank and more
Diffraction of white light into rich beautiful colors - subtraction spectrum showing yellow, magenta, and cyan - diffraction of red laser light from a single hair - interference of diffracted red laser light from two hairs. See this and more at the annual VIP spring meeting on April 24th at the University of Virginia.
The morning session will feature a share session. Dr. Richard Lindgren, retired Research Professor of Physics at the University of Virginia, will discuss and demonstrate some diffraction labs that you can do. Please consider bringing an activity (related to diffraction or not) that you can share in 5-10 minutes.
After a lunch on your own in town or on campus, the afternoon will center on a make and take session and possible demonstration from UVA's physics lab. Do you have ideas for cool things we can make on a limited budget? Please feel free to share ideas on the VIP listserv or directly to greg.matthes@fcps.edu
Also consider joining VIP members in making a presentation at the 2010 VAST Professional Development Institute in Hampton Roads next November. Last November twelve VIP members presented in ten different sessions.
Greg Matthes, Physics Teacher, Robert E. Lee
|
| The Cheap Chain Reaction Demo |
by Tony Wayne
Background
We have all see demonstrations of a chain reaction. In a chain reaction one molecule decays and in an instant pieces of this molecule hit other molecules causing them to decay. This multiplication escalates the rate of decay into an uncontrollable process.
“Nuclear Chain Reaction with Balls :: Physikshow Uni Bonn”, Posted by
TheTvelvethMonkey January 6 2007, http://www.youtube.com/watch?v=ORqc1x3_Evg
“Touchstone-Mousetraps,” posted by designworks, July 06, 2007, http://www.youtube.com/watch?v=JxzPN-vdP_0:
These demonstrations usually involve a large box with acrylic sides and maybe 100’s of mousetraps and ping pong balls. When you think about doing this in a high school setting, the typical teacher wonders how will he or she set all the traps, place all the balls on the traps, and go to the bathroom in between classes. Then comes the question of where to store a box that is 6 feet by 3 feet by 2 feet. Here is an inexpensive alternative. It is not as dramatic …until someone sets it off by accident.
Materials
- The box a “case” of paper comes in -20 reams of paper.
- A piece of screen or acrylic sheet large enough to cover the top of the box. (The screen is much cheaper.)
- 16 spring type mouse traps. ..from ebay, Walmart, etc
- 16 pingpong™ balls … from ebay
Construction and Procedure
With the lid off, set and place the traps. Set the traps in the box in 4 rows and 4 columns. Be careful how you hold them. Hold b the edges such that if the trap snaps, the metal bar will move away from your fingers. Once it is in the box. Gently push it into place with a pencil.
When is it time to place the PingPong™ balls, have the student place them in place. If you are lucky, they will set them off while placing them on the traps it is an experience to remember. If you have a small video camera, like a flip camera, set it u on one side of the box and have the students stand opposite the camera. This way when the traps go off, accidentally, you’ll have a video the look back on to see what actually happened.
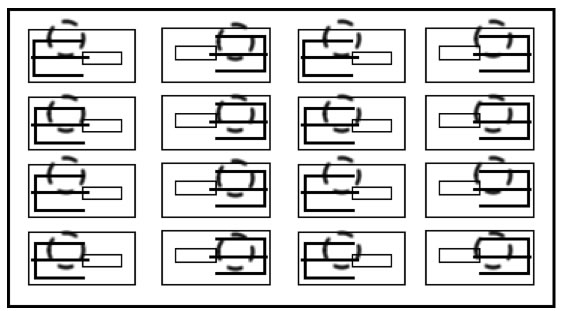
|
Overhead view of the mousetraps in the paper box.
Notice how each pair of mousetraps face each other.
|
If the traps have not gone off, place the screen over the top of the box such that is covers all but about 2 inches. Toss a ball into the box through this open end and watch the reactions. Then show the students one of the videos above to see a really big reaction.
| An Action Reaction "Punny" Example |
by Tony Wayne |
We are always trying to make create “hook: so students can remember what we are trying to teach. Here is one that I’ve enjoyed.
Background
Newton’s Third law of motion says that for every action there is an equal and opposite reaction. This means that all forces come in pairs.
Materials
|
- “plastic” covered pear from a craft store like “Michaels.”
- Hacksaw blade
|
- Manila folder or card stock
- White glue
- Thick Marker (Like a “standard” Sharpie)
|
|
Construction
To emphasize this I have gone to craft store that sells fake fruit and purchased a pear. This pear has a plastic outer shell, a Styrofoam™ body and a weigh in the center to make it sit like a pear. Using a hacksaw blade the pear is cut in half. When the blade reaches the center of the pear. Stop cutting and think about how to cut around the weight. Once you have successfully cut around the pear weight in the middle pull the pear apart. The weight will be stick on one of the halves. Pull it out and discard it. Now you have two pear halves with a hole in the middle of each half. Place the pear half, flat side down, on a piece of manila folder or card stock and trace its outline. Cut out the outline. Do this for each half. On one piece of manila folder write “Action Force” with an arrow pointing “up.” On the other piece write “Reaction Force” with an arrow pointing down. Put the two pair halves in a lunch box of brown lunch bag.
|
Presentation:
After discussing Newton’s 3rd law, say to the class, “I have physical proof of Newton’s 3rd law in my lunch.” From a hidden location pull out your lunch box. Reach in and carefully pull out the pear in one piece. Then separate the two halves and show the students. Pause for a moment and say, “See? Forces do come in pairs.” They will groan. But they will also remember that “forces come in pairs.”
|
Why You Should Teach Particle Physics |
by Greg Matthes
A unit on particle physics offers the opportunity to combine basic concepts such as conservation of energy, momentum , and charge along with conservation laws that only get discussed in the realm of particle physics (baryon #, lepton # and more). Principles from special relativity, E&M, thermo, optics and mechanics are applied across a panorama from particles to enormous accelerators and from the big bang to cosmic background radiation. While names like virtual particles, eight-fold way, quantum tunneling seem both strange and beautiful the concepts behind the names can be understood by students at the completion of a 1st year physics curriculum.
From a social aspect the impact of particle physics research has a tremendous impact on our lives. “Some applications of particle physics-the superconducting wire and cable at the heart of
magnetic resonance imaging magnets, the World Wide Web—are so familiar they are almost clichés. But particle physics has myriad lesser-known impacts. Few outside the community of experts who study the behavior of fluids in motion have probably heard of the particle detector technology that revolutionized the study of fluid turbulence in fuel flow. Selected examples from medicine, homeland security, industry, computing, science, and workforce development illustrate a long and growing list of beneficial practical applications with contributions from particle physics” and can be found at http://www.fnal.gov/pub/science/benefits/
Where to get started: CERN’s site: http://particleadventure.org/index.html This site has a myriad of information and useful links and is excellent for introducing historical perspectives and basic nomenclature. More advanced material is also available; try the link to http://pdg.lbl.gov/fireworks/intro_eng.swf for a fun interactive exercise with Feynman diagrams. [you may have to use Firefox to access this site from your school].
Getting real data to process: Canada’s national accelerator facility, TRIUMF, has tutorials on the cyclotron and data on momentum and relativistic mass of a muon: http://www.triumf.info/public/students/videos/
Want a real treasure hunt to apply all the conservation laws? Try bubble chamber exercises. Tutorials, exercises and an excellent power point are at http://teachers.web.cern.ch/teachers/archiv/HST2005/bubble_chambers/BCwebsite/index.htm
Closer to home try Hampton University’s http://cosm.hamptonu.edu/tirpwiki/doku.php?id=start:part:class:bubble for more exercises that students find engaging – real particle “who-done-its” that are fun and come in a variety of levels.
Not sure how to start? The Hampton University Quarknet group http://cosm.hamptonu.edu/tirpwiki/doku.php?id=start:disc has meetings, training and various opportunities to get involved.
What a way to conclude a 1st year physics course!
greg.matthes@fcps.edu
|
Google Apps and Google Docs in the Lab with Web 2.0 |
by Tony Wayne
The “Web 2.0” a dynamic web where the content is generated by the users and is done in a collaborative manner. Google docs and Google Apps can help to build this mode l in your classroom. The big difference beween the two is the number of people that can share a document at the same time. In Google docs when 16 people share the document at the same time, all users loose contact with the document. With Google Apps for Education, this number is much higher, 150.
Labs/Activities
Tools: Google Apps for Education http://www.google.com/educators/p_apps.html
YouTube: http://www.YouTube.com
Jing: http://www.jing.com
Google Apps for education is used to build a collaboration model among the teams working on a lab or activity. “Google Apps for Education” is a suite of tools that include Google Docs among many others. Google Docs is a word processor with some graphics capabilities. Google Docs allows multiple users to edit the same document at the same time. This is called sharing. A lab consists of two Google Docs documents. The first document is titled with the name of the lab or activity, e.g. “Work from a Graph.” This document contains everything but space for student responses. It includes the background, objectives, procedures, questions and conclusion. When every possible, animated gifs showing what is happening will be in this document. Depending on the lab, this file may contain a video link stored on YouTube. The videos are created with the movie function of a digital camera and uploaded to YouTube. This video might demonstrate how to use their calculator to solve something special –like solving a set of simultaneous equations using the “rref” function. The video might be a screencast showing how to use a piece of software or the basic equipment setup. “Jing” can be used to make the screencast. This Google Doc is shared with my students in such a way that they can only view it without editing it.
The second document is for student editing. For organizational purposes, it is titled with an acronym for the lab. For example the “Work from a Graph” lab might be called “WG.” It will act as a template with question numbers and drawing spaces. Some of the drawing spaces may contain partial drawings where the students fill in the rest. This is the only paper I see for grading purposes. When this document is shared with my students, it is shared as a view only document. Students make a copy of the file for editing purposes. When they make a copy of the file, the file becomes editable. All students are instructed to make there responses in a blue font. This makes grading much easier. The file copy has a special name. The name begins with the class period, space, file acronym, space, followed by the last names of the student group in alphabetical order. For example Jim Kirk, JeanLuc Picard and Katherine Janeway are in third period. Their “Work from a Graph” lab is called, “3 WG Janeway, Kirk, Picard.” This document is now shared among the group members and the teacher. This file also includes an ownership section at the top. The students are to fill out who has each responsibility and who is double-checking. When the students are filling out the document, each person is working on the file at nearly the same time. It cuts down on overall lab completion time. The students collaborate on the file’s contents and talk much more while answering the questions. They see how the sections depend on each other.
Students can print the document or you can grade it electronically using the comment function in Google docs.
|
Spectral Lines and the Discovery of the Composition of the Sun and Stars |
“They said Iron Agaien”
by Mary Fuller and Andy Jackson
This is a series of experiments that Mary and I developed during a summer workshop at Greenbank WV. I will bring the lab sheets and further details to the spring meeting at UVA. This is designed as a physics lab with the assumption the students have seen spectral lines before in chemistry class. The activities use the spectral lines as an engaging hook to teach the spectrum of visible light, the presence of ultraviolet and infra red radiation and to use some interesting history to teach about the nature of science.
Engage
Bright line spectra using flame tests and high voltage gas tubes viewed through diffraction grating.
Explore
Students will draw (using color pencils or crayons) a continuous spectrum produced by an incandescent bulb and selected bright line spectra.
Explain
Lecture on the nature and cause of bright line spectral lines.
Expand to include UV and IR through various demos. (glow in the dark activated by UV, UV beads, IR w/ CBL temperature probes)
Explore/Extension
Students will be instructed to go look at sunlight reflecting off of a white surface and carefully observe and draw what they observe. The student will be asked to theorize why the dark lines of the absorption spectrum are present.
Explain
Lecture/demo Cecilia Payne-Gaposchkin’s contributions in this field.
Resources and teacher notes
Equipment
Gas discharge tubes & high voltage power supply, Bunsen burner, salts, diffraction gratings, and GOOD spectrometer. ( This variety - http://sciencefirst.com/product_info.php?products_id=445&osCsid=8159ecab631190a0c9fad4fa77e48b7c for $7.95 each – qualitative or this http://sciencefirst.com/product_info.php?products_id=403&osCsid=8159ecab631190a0c9fad4fa77e48b7c for $28.95 much more quantitative)
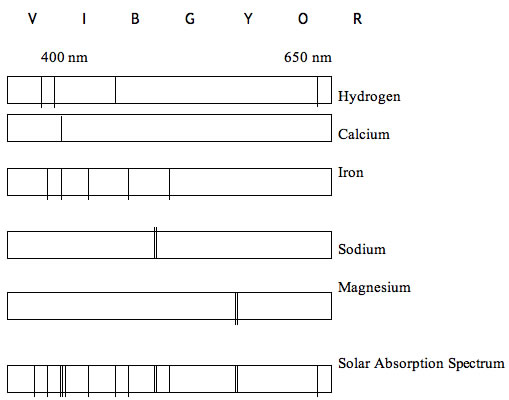
Students love looking at emissions spectra. A simple worksheet with a rectangle drawn for each sample is all the paper work you have to prepare. Students should label wavelengths and color the full spectrum by looking at light from an incandescent light bulb. Interesting topics to discuss are what is the wavelength of the reddest red and the “violetest” violet that you can see? If you have the gas discharge tubes and transformer available, have students look at and color spectra for Hydrogen, Helium, Sodium, Oxygen, and Mercury. Look at emissions from flame tests for Sodium, Magnesium and Iron.
After some time looking and coloring, it is time to learn what makes the pretty colors. I explain the simple spectrum of Hydrogen and “hand wave” the rest. In Hydrogen the one electron can be pumped up to, and fall down from, a variety of energy levels producing the discreet separate color lines. The absorption spectrum for our sun can be seen by looking at a bright white sunlit concrete surface. You have to find the right balance between shade for the viewer’s eye and brightly lit surface. Of course YOU NEVER LOOK DIRECTLY AT THE SUN. The absorption spectra is caused by the light traveling through the outer gasses of the sun. These gasses absorb the same frequency they would emit. From these absorption lines you can surmise the elements present in the outer gasses of the sun.
Why the misspelled title? Because when all the other scientists were claiming the sun was made primarily of Iron, Cecilia Payne-Gaposchkin saw something different. “They Said Iron Agaien” but she saw “they said iron agaien”. (E=mc2: A Biography of the World’s Most Famous Equation, David Bodanis)
Good Spectrum Resources
E=mc2: A Biography of the World’s Most Famous Equation by David Bodanis
Seeing and Believing: How the Telescope Opened Our Eyes and Minds to the Heavens by Richard Panek
http://www.phys.virginia.edu/CLASSES/252/spectra.html - Dr. Michael Fowler of UVA lecture notes on the history of our knowledge of spectra
http://cannon.sfsu.edu/~gmarcy/cswa/history/cecilia.html - History of Women in Astronomy by the Astronomical Society of the Pacific detailing some of Cecilia Payne-Gaposchkin’s contributions.
http://www.cavendishinstruments.com/sapphire.htm for a beautiful color version of the diagram below.
|
| |
|