What’s the Point, Currie?
Presenter: Andy Jackson
Harrisonburg City Public Schools, ajackson@harrisonburg.k12.va.us
Va. SOL:
PH.8 The student will investigate and understand that energy can be transferred and transformed to provide usable work. Key concepts include
- transformation of energy among forms including mechanical, thermal, electrical, gravitational, chemical, and nuclear; and
- efficiency of systems.
PH.12 The student will investigate and understand how to use the field concept to describe the effects of gravitational, electric, and magnetic forces. Key concepts include
- inverse square laws (Newton’s law of universal gravitation and Coulomb’s law); and
- operating principles of motors, generators, transformers, and cathode ray tubes.
PH.13 The student will investigate and understand how to diagram and construct basic electrical circuits and explain the function of various circuit components. Key concepts include
- Ohm’s law;
- series, parallel, and combined circuits; and
- circuit components including resistors, batteries, generators, fuses, switches, and capacitors.
Topic/Concept
The Currie temperature will be determined for a piece of steel wire through the use of ohm’s law and the relationship between temperature and resistance of a metal wire.
Materials
All included in the write up
Safety Considerations
The steel wire will become red-hot in this experiment and will blister skin upon contact. It is very possible that the wire could break and in doing so there is a chance a piece could be thrown, therefore eye protection must be used.
Presentation
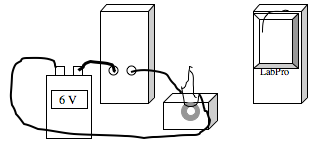
The Currie point for steel is determined through experiment utilizing a simple circuit, ohm’s law and temperature/resistance relationship for metals.
What’s the Point, Currie?
When a material that is ferromagnetic (strongly attracted to a magnet) is heated to a certain temperature it becomes no longer ferromagnetic but only paramagnetic (very weakly attracted to a magnet – like water!). This temperature is known as the Currie point or Curie temperature. In this experiment we will pass a large current through a small length of steel wire until it gets red-hot. You will closely monitor the voltage across and the current through the wire. We will then apply Ohm’s law to calculate the resistance of the steel wire when it is so hot that it is no longer ferromagnetic. We will know the precise moment when we’ve reached this temperature since a magnet will no longer stick to the wire. To determine the temperature of the wire when it transitioned from ferromagnetic to paramagnetic we will utilize the fact that metal wire’s resistances increase linearly with temperature.
Objective – utilize knowledge of simple circuits and data gathering with current and volt meters to determine the Currie point of a sample of steel wire.
Materials
- 6 Volt lantern battery
- 3-4 cm piece of thin steel wire – individual strand from braided picture hanging wire
- Ammeter capable of up to 2-3 Amps
- LabPro with Vernier Voltage probe
- Assorted hook up wires two with alligator clips
- Small strong magnet
Procedure – Construct the circuit shown in the diagram below. The A is the multimeter in the 10 A setting. The V is the LabPro measuring Voltage. The gray cylinder is the magnet.
Procedure
- Build the circuit but do not make the final connection to the battery.
- Make the steel wire into a ‘U’ that hangs down vertically. Attach the magnet to the loop of steel wire.
- Make sure the ammeter is in the setting that will allow it to measure up to at least 3 A.
- Set up the LabPro to collect voltage every 0.10 s for 10 seconds.
- Put on eye protection
- CAUTION – WIRE WILL BECOME RED HOT. IT WILL BLISTER SKIN UPON CONTACT.
- Start the LabPro and as soon as it begins collecting data, make the final connection in the circuit.
- Record the current from the ammeter as soon as the final connection is made.
- Record the current as soon as the magnet falls off the wire.
- As soon as the magnet falls off the steel wire loop and the final current is recorded, disconnect battery.
Trouble shooting
- It is very possible for the wire to get so hot that the wire burns in two and the magnet falls nearly simultaneously. If this occurs, try again with a slightly longer section of wire between the alligator clips.
- It is very possible for the wire to not get hot enough to reach the Currie point. If this occurs, try again with a slightly shorter section of wire between the alligator clips.
Analysis
- From the graph determine the Voltage across the steel wire as soon as the circuit was connected and at the moment the Currie point was reached.
Vi ___________ Vcp ____________
2. How well do you know these two voltages? Any reason to doubt their accuracy?
3. What was the amount of current passing through the steel wire as soon as the circuit was connected and at the moment the Currie point was reached?
Ii ___________ Icp ____________
4. How well do you know these two currents? Any reason to doubt their accuracy?
5. Determine the resistance of the steel wire at the moment the circuit is first connected and at the moment the Currie point is reached. Show your work.
6. The equation Rf = aRoDt + Ro describes how a metal changes resistance as a function of temperature. a= 0.005671 c-1 for Iron and 0.003 for steel. The value for steel can vary quite a bit depending on the exact composition. Use these two values and this equation to determine the Currie point (or actually in this case a temperature range) for the steel wire. And, as always, show your work.
7. What aspect of this experiment introduces the greatest uncertainty? Why?
Teacher Tips Regarding Lab
This lab could be greatly improved if a good source of pure iron wire thin enough to reach the Currie temperature easily could be found. Then a very specific accepted value would be known. As is, this lab could be made more accurate by conducting an experiment to determine the value of a.
|